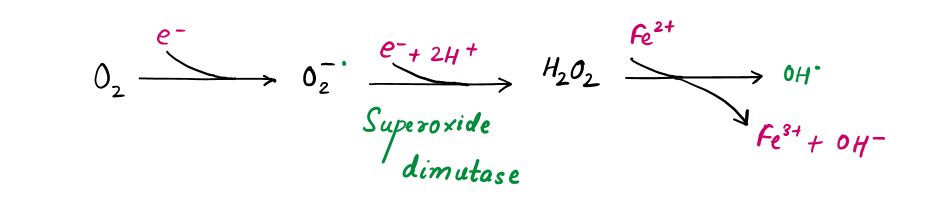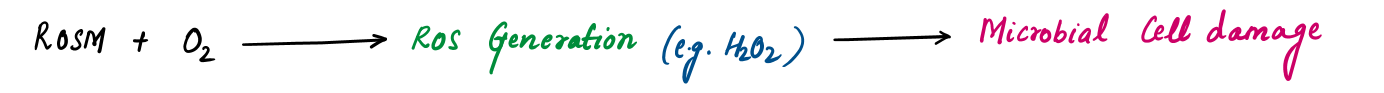1.Introduction
1.1 "Tulsi Pujan Diwas" and their importance in Indian tradition
Tulsi Pujan Diwas, a sacred observance dedicated to sacred herbs named as Tulsi plant, is celebrated annually on 25th December in India. This day is a time for people to honor the Tulsi plant, which holds a significant place in spiritual and cultural practices and health. The day is marked by prayers, rituals, and offerings, where devotees worship the Tulsi plant, seeking blessings for health, prosperity, and spiritual well-being.
In Indian culture, Tulsi is often regarded as the "Mother of Herbs."
Tulsi plants are commonly found in every household, often planted in a special courtyard or garden. The act of watering and caring for Tulsi is seen as a form of devotion.
It is also beneficial for its medicinal and therapeutic properties, known for improving both physical and mental well-being, and acts as a remedy for a variety of ailments ranging from common colds to more complex chronic conditions like stress, inflammation, and cellular damage due to oxidative stress.
1.2 Purpose of Article: Tulsi as a potent adaptogen
The purpose of this article is to enlighten the scientific perspective of Tulsi regarding many health and environmental benefits, explaining how its biochemistry contributes to the healing powers attributed to it in traditional practices.
By bridging tradition with modern science, we can see how Tulsi acts as both a spiritual symbol and a natural remedy. This article will explore different phytochemical compositions of tulsi, how these compounds interact within the human body and their impact on the environment through reaction mechanisms.
We are amid a global pandemic of obesity, diabetes, cancer, dementia, depression, and other chronic diseases caused by modern lifestyles and their associated lack of physical activity, high intake of sugar, fat, salt, alcohol, and tobacco, and exposure to a toxic cocktail of industrial chemicals. The solutions to this current health crisis are therefore more likely to be found in the homes and behaviors of individuals than in medical clinics, hospitals, or pharmacies.
2. Key Bioactive Components (Phytochemicals) in Tulsi
a. Eugenol
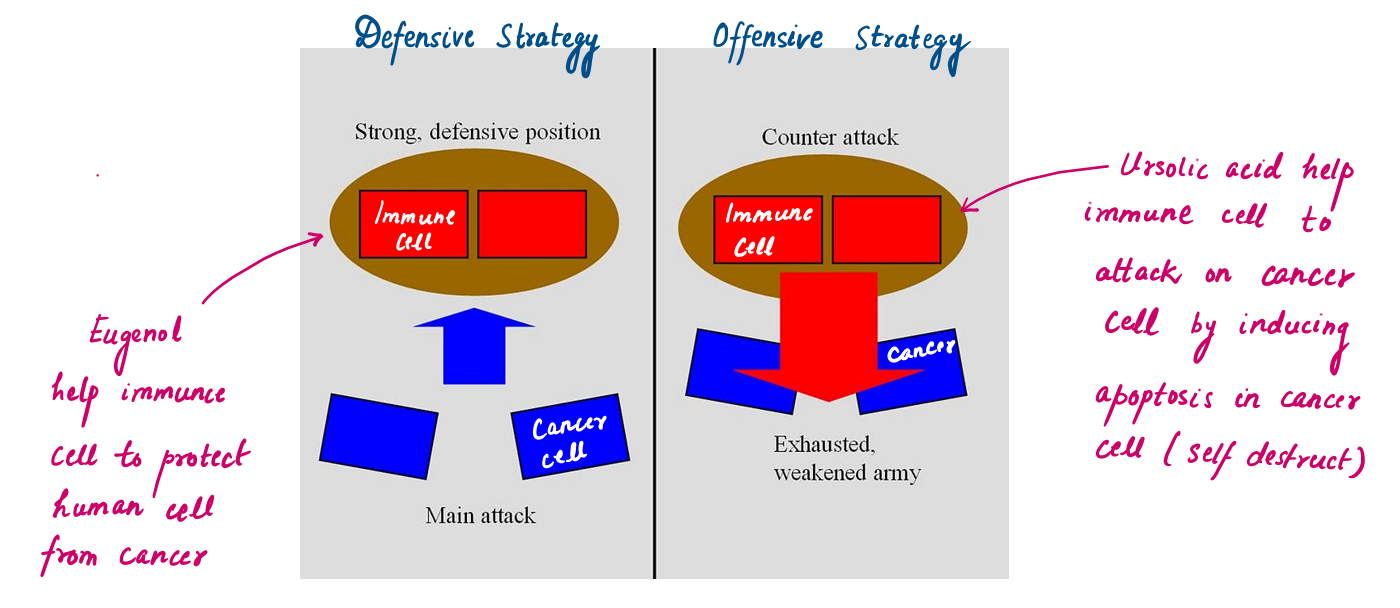
- A phenolic compound contributing to its anti-inflammatory and analgesic properties. It is a main component of essential oils.
- Anti-inflammatory, antimicrobial, and antioxidant properties.
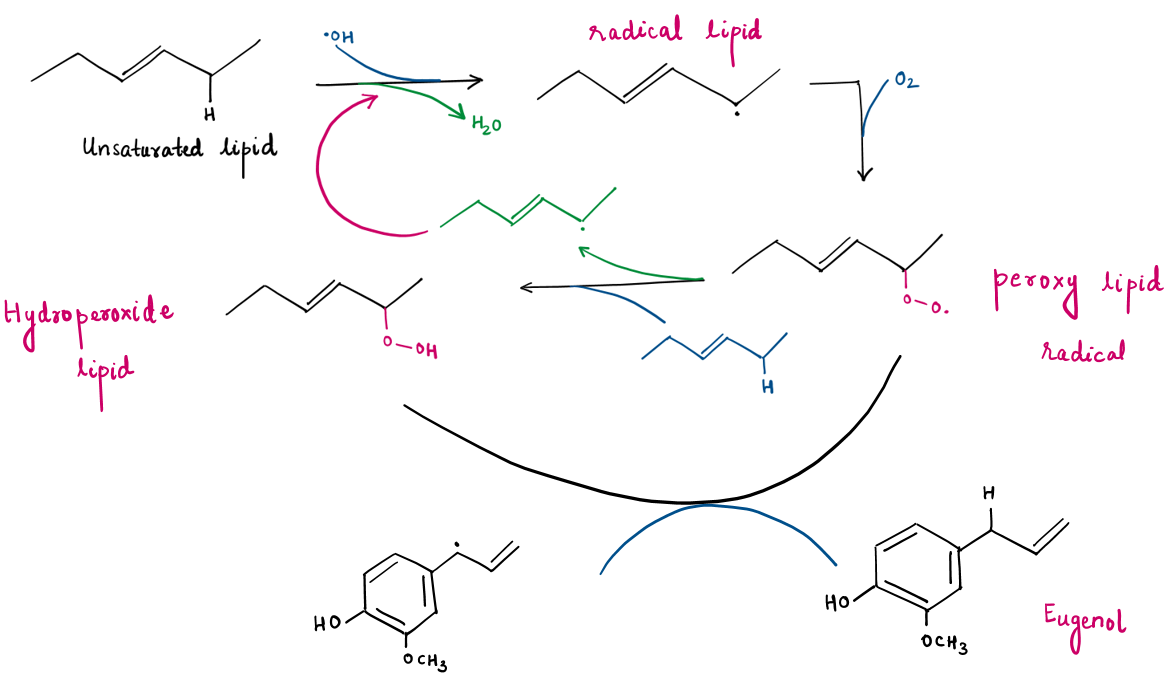
- Reaction Mechanism (Inhibition of Lipid Peroxidation) :
Lipid peroxidation involves free radicals attacking lipid membranes, leading to oxidative stress, which can alter the physical properties of cellular membranes, leading to cardiovascular diseases, asthma, and kidney damage.
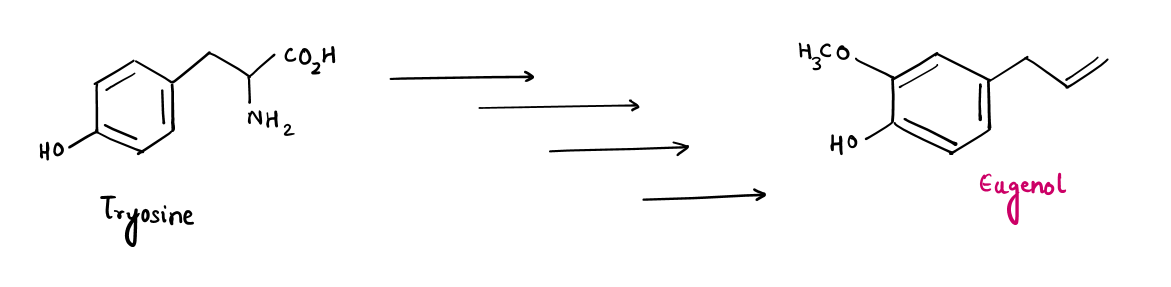
Bio-synthesis of Eugenol via Tyrosine (alpha amino-acid)
Eugenol scavenges free radicals by donating a hydrogen atom, breaking the lipid peroxidation chain, and reducing oxidative stress.
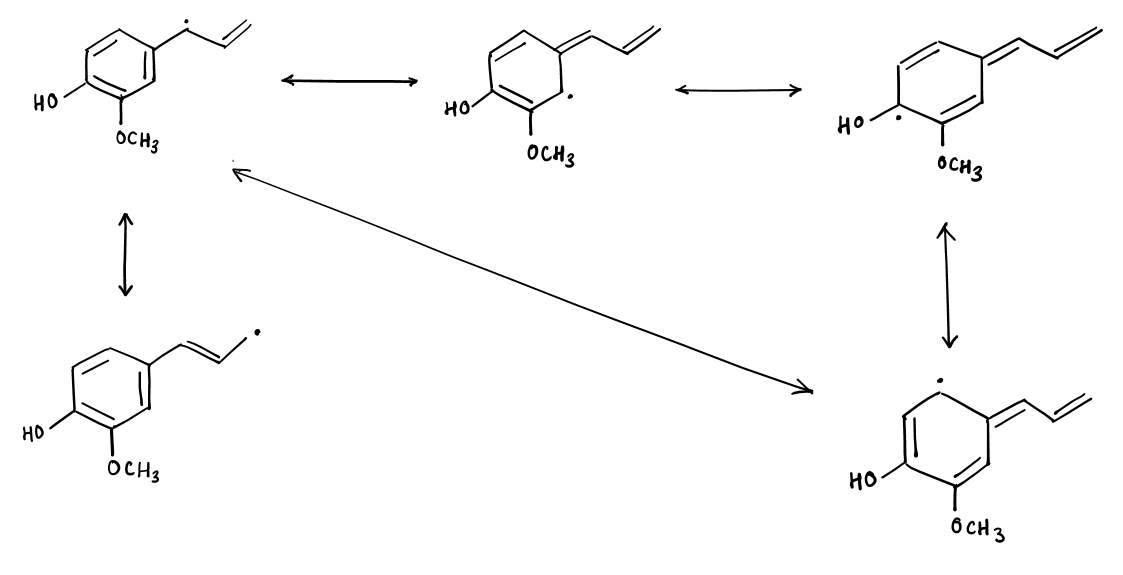
Antioxidants such as Eugenol accept the free radical reaction energy and spread the reaction potential within the phenol ring structure by the resonating double bonds. This significantly lowers the free radical reaction potential of the antioxidant. So, the Eugenol radical cannot be involved in the propagation process and the lipid oxidation process stops there.
The resonance-stabilized structure of Eugenol to inhibit further radical reaction of lipid
Anti-microbial and anti-inflammatory properties of Eugenol :
Eugenol and other essential oils disrupt microbial cell membranes, causing leakage of ions and cellular content, leading to cell death. Recent research highlights its potential in combating viral infections, including SARS-CoV-2.
Antiviral Properties:
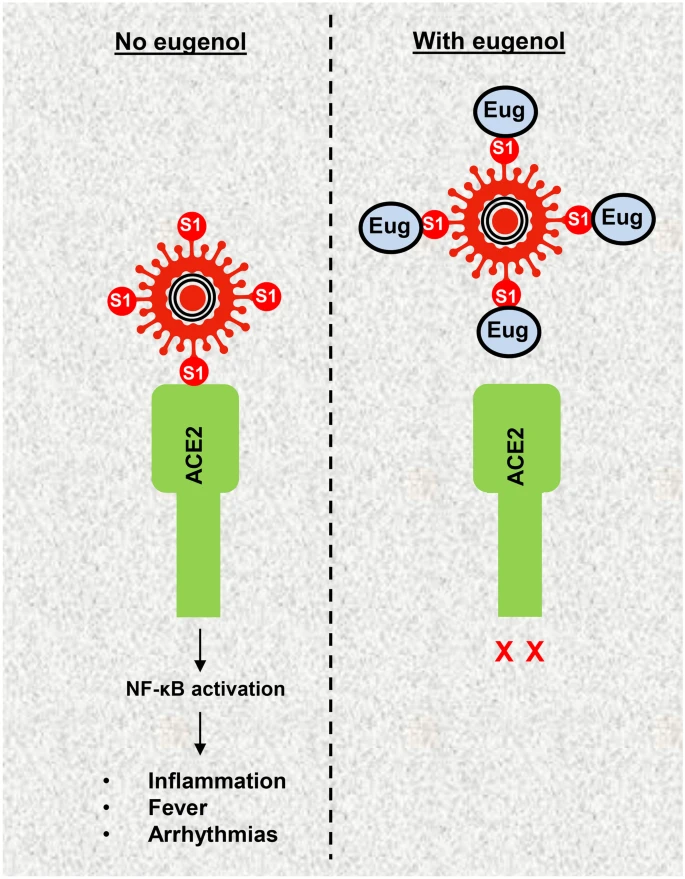
Eugenol has demonstrated the ability to inhibit the entry of pseudo-typed SARS-CoV-2 into human cells by disrupting the interaction between the virus's spike protein and the ACE2 receptor. This disruption reduces the virus's capacity to infect host cells.
However, according to recent experimental studies, other phytochemical components of tulsi such as ursolic acid, oleanolic acid, and β-caryophyllene remain unable to hinder the interaction between spike S1 and ACE2.
Anti-Inflammatory Effects:
In human lung cells, eugenol has been shown to suppress the activation of NF-κB, a protein complex that plays a role in inflammatory responses. This suppression leads to decreased expression of pro-inflammatory cytokines such as IL-6, IL-1β, and TNFα, which are often elevated during infections.
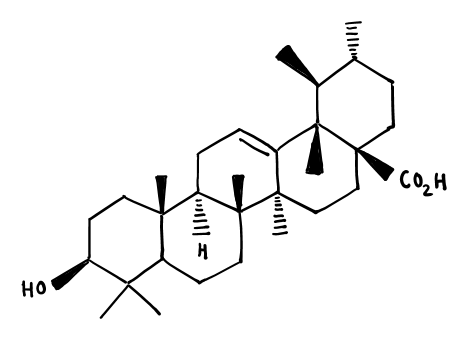
b. Ursolic Acid
- Ursolic acid is a pentacyclic triterpenoid in which green tulsi leaves contain more ursolic acid (0.478 %) than black tulsi leaves (0.252 %), having many biological activities, including anti-inflammatory, anti-cancer, and anti-tumoral properties.
The molecular structure of Ursolic Acid
Anti-cancer and anti-tumoral properties of Ursolic acid with Mechanism :
Ursolic acid shows significant anti-cancer properties by targeting cancer cells or controlling potential possibilities before turning into cancerous cells in various ways such as :
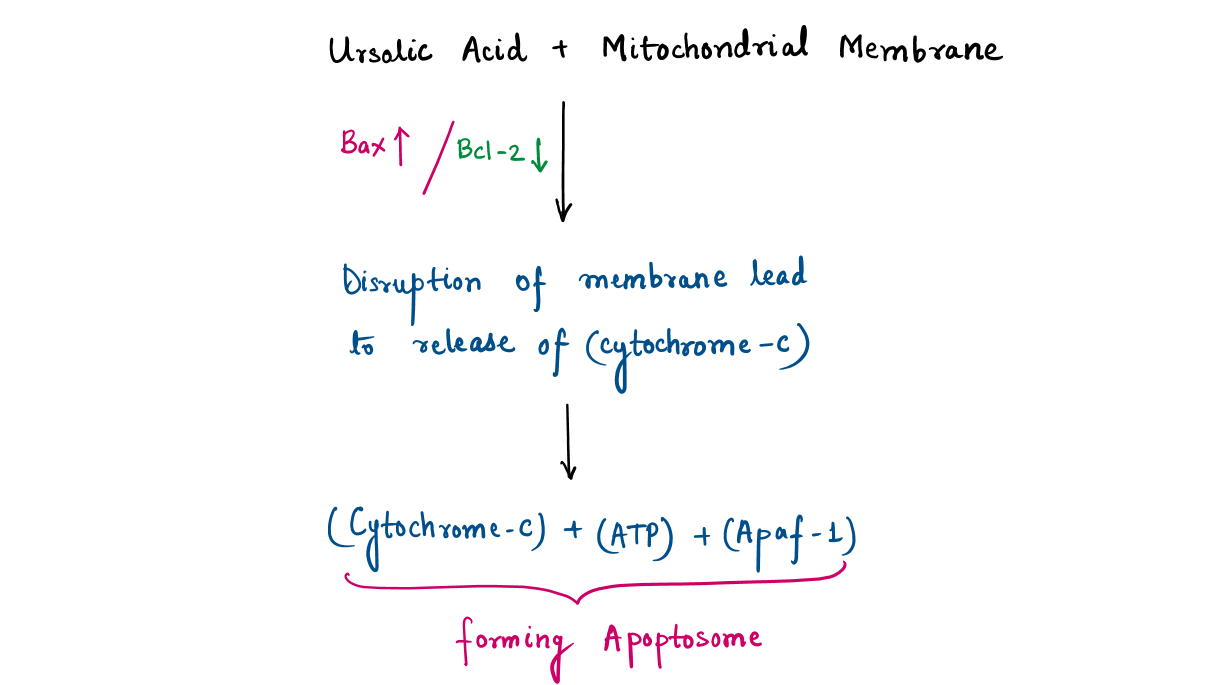
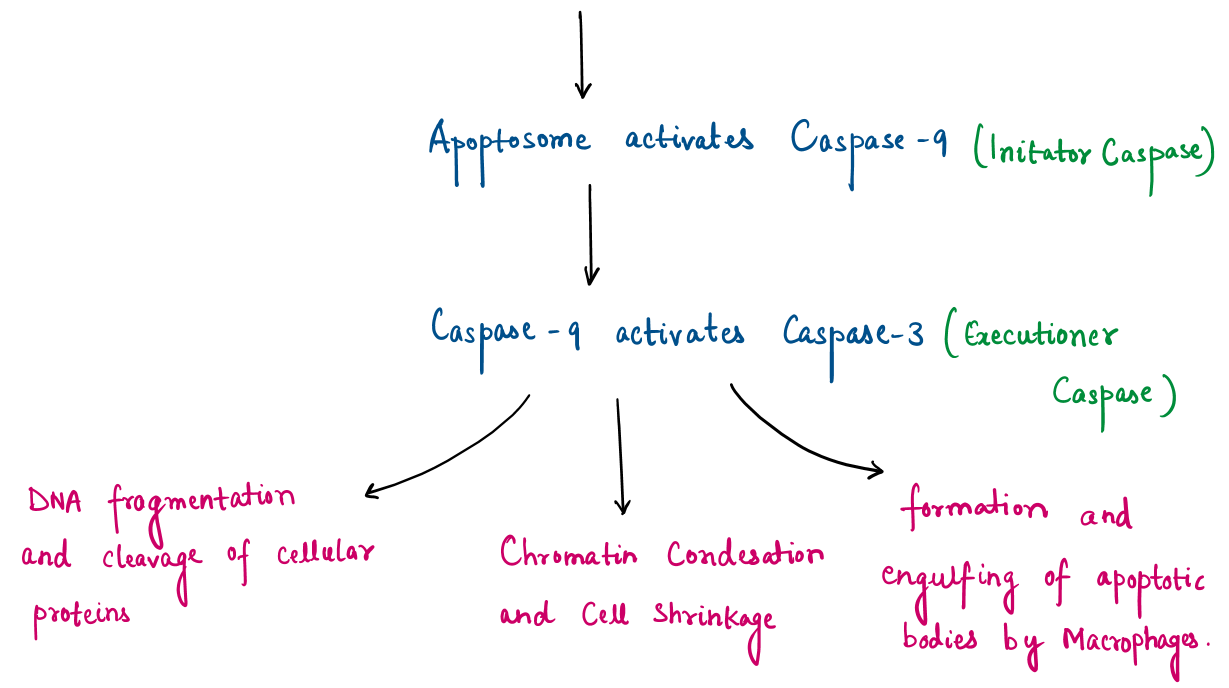
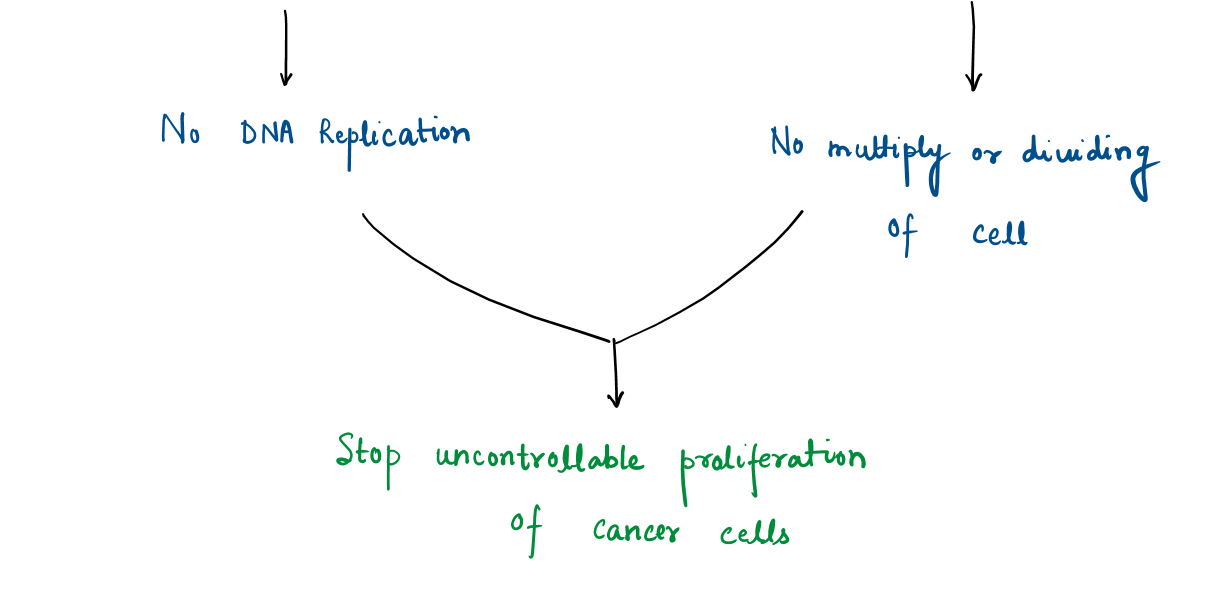
- Inducing apoptosis (the biological process leading to the self-destruct of cancer cells) by activating enzymes helps in executing apoptosis (Caspase) or blocking signaling pathways (like STAT3 pathway) which helps cancer to grow.
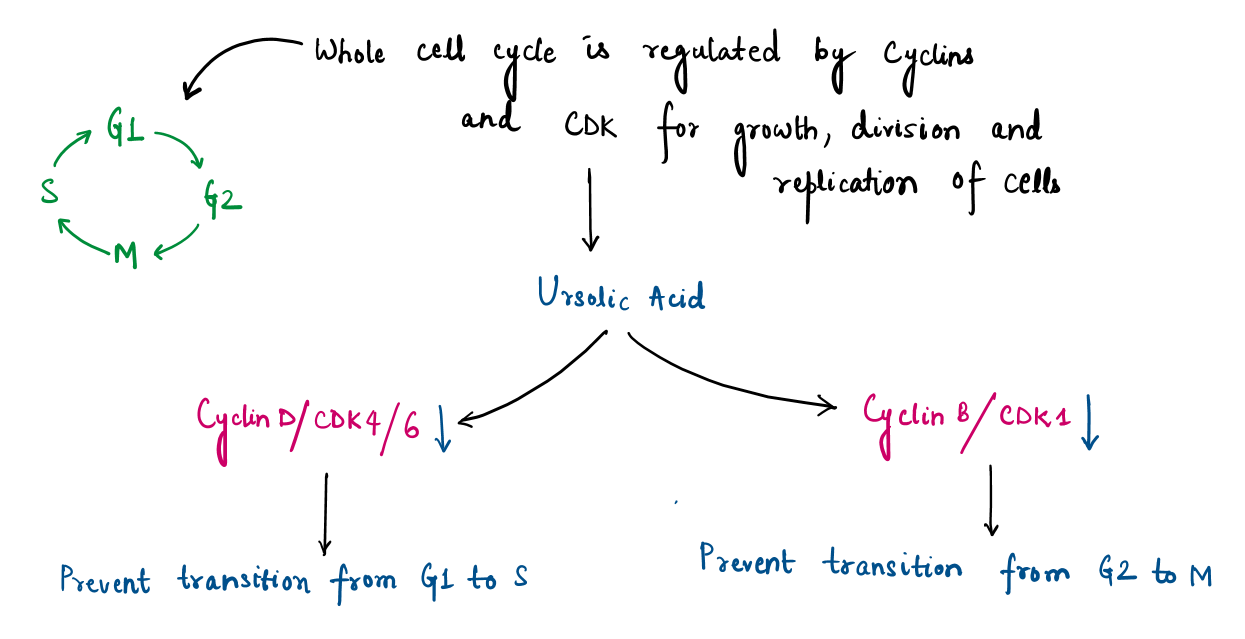
- suppress uncontrollable cell division of corrupted or mutated cells at specific stages of the cell cycle (e.g., G1/G2 phase).
- prevent the spread of cancer (metastasis) by suppressing MMPs (MMPs is an enzyme responsible for breaking down tissue barriers, allowing cancer cells to invade other organs) or inhibiting EMT (EMT is a process of transition where cancer cells become more mobile and invasive)
- inhibit an angiogenesis phenomenon (the formation of new blood vessels to supply nutrients for growth and nurturing of tumor), starving the tumor of its nutrient supply.
- Chronic inflammation is linked to cancer progression. Hence, Ursolic acid is observed to reduce pro-inflammatory cytokines (like IL-6 and TNF-α) or suppress inflammatory pathways, including COX-2 and NF-κB.
-
Ursolic Acid induces apoptosis in cancer cells by increasing ROS (Reactive Oxygen Species), leading to damage of DNA, protein, and lipid. ROS are byproducts of normal cellular metabolism (e.g., in mitochondria) but can accumulate during oxidative stress.
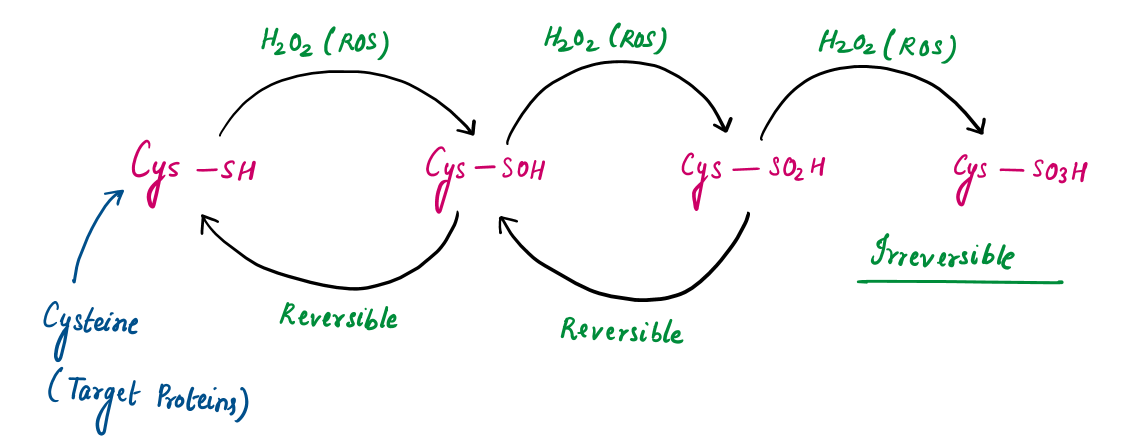
To demonstrate the chemical reaction of ROS with a protein, let’s choose Cysteine as the target amino acid residue. This is because cysteine residues in proteins are highly reactive toward ROS due to their thiol (-SH) groups, which can undergo oxidation.
Step-1 : Ursolic acid disrupts the mitochondrial electron transport chain, leading to the formation of hydrogen peroxide (H₂O₂) and hydroxyl radicals (OH•) such as :
Step-2: The thiol (-SH) group in cysteine reacts with ROS, resulting in a series of oxidative modifications:
High levels of ROS irreversibly oxidize sulfinic acid to sulfonic acid (-SO₃H), leading to irreversible damage.
Consequences of Protein Oxidation by ROS will be protein misfolding, loss of enzymatic activity, protein aggregation (due to irreversible modifications).
For this point, we can conclude that ursolic acid-generated ROS modifies proteins, contributing to cancer cell death while sparing healthy cells that have better antioxidant defenses.
Comment on the effect of ROS on other amino-acid
Amino-acid | Reactive Group | ROS Damage Mechanism | Effect |
Cysteine (Cys) | Thiol (-SH) | Oxidation to sulfenic, sulfinic, or sulfonic acid | Disulfide bond disruption |
Methionine (Met) | Sulphur atom | Oxidation to sulphoxide/ sulfone | Structural damage |
Tryptophan (Trp) | Indole ring | Oxidation to kynureine derivatives | Signal disruption |
Histidine (His) | Imidazole ring | Oxidation to imidazolone | Enzyme inactivation |
Proline (Pro) | Pyrrolidine ring | Oxidation to carbonyl derivatives | Structural damage |
Lysine (Lys) | Amino group | Formation of allysine, cross-linking | Structural damage in collagen |
Arginine (Arg) | Guanidinium group | Oxidation to glutamic semialdehyde | Protein misfolding |
Phenylalanine (Phe) | Benzene ring | Hydroxylation | Moderate damage |
Serine (Ser) | Hydroxyl group | Oxidation to aldehyde | Minimal damage |
Threonine | Hydroxyl group | Oxidation to aldehyde | Minimal damage |
Glutamine (Gln) | Amide group | Carbonyl formation | Protein misfolding |
Asparagine (Asn) | Amide group | carbonyl formation | Protein |
Glutamic Acid (Glu) | Carboxylic acid | Mild carbonyl damage | Moderate damage |
Aspartic acid | Carboxylic acid | Mild carbonyl damage | Moderate damage |
Glycine (Gly) | No reactive side chain | Targets peptide bonds | Relatively resistant |
Alanine (Ala) | No reactive side chain | Targets peptide bonds | Relatively resistant |
Valine (Val) | Hydrophobic side chain | Minimal oxidation | Minimal damage |
Leucine (Leu) | Hydrophobic side chain | Minimal oxidation | Minimal damage |
Isoleucine (Ile) | Hydrophobic side chain | Minimal oxidation | Minimal damage |
Tyrosine (Tyr) | Phenol group | Dityrosine cross-links, quinone formation | Protein Aggregation |
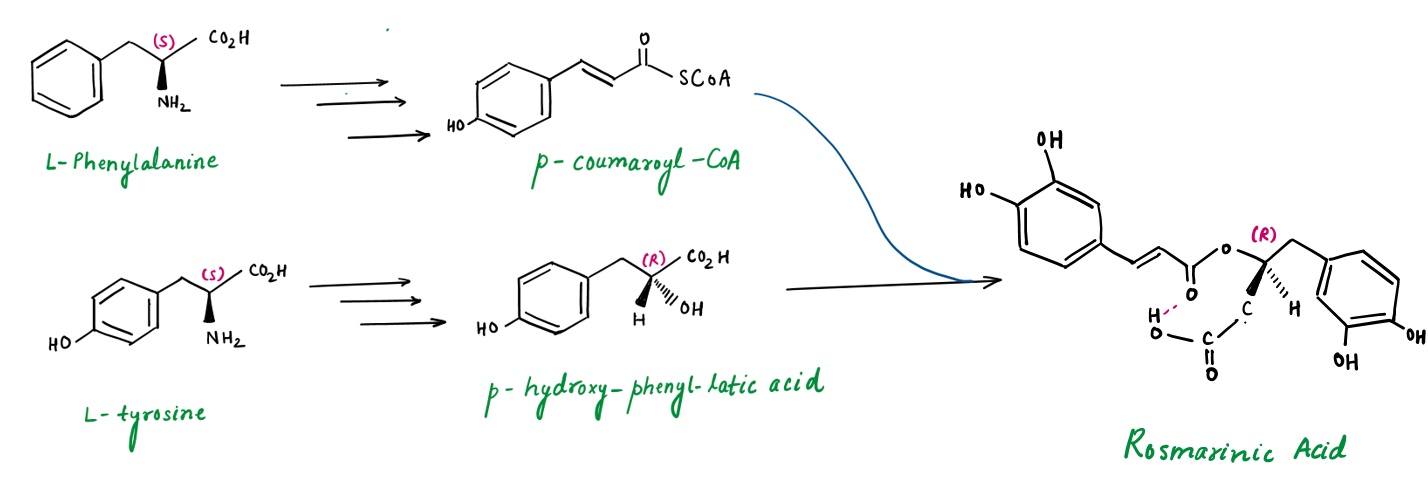
c. Rosmarinic acid
Rosmarinic acid, a polyphenol in Tulsi (Ocimum sanctum), providing various therapeutic benefits while complementing other compounds like ursolic acid and eugenol.
The biosynthesis of Rosmarinic Acid
Unique feature of Rosmarinic acid :
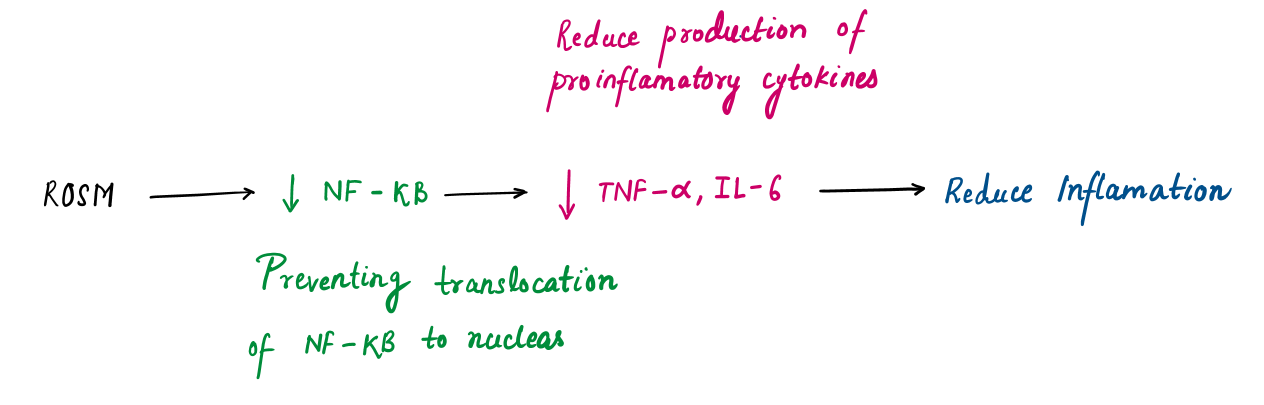
- Anti-inflammatory: It inhibits COX-2 and NF-κB pathways, reducing cytokines like IL-6 and TNF-α, making it effective against chronic inflammation.
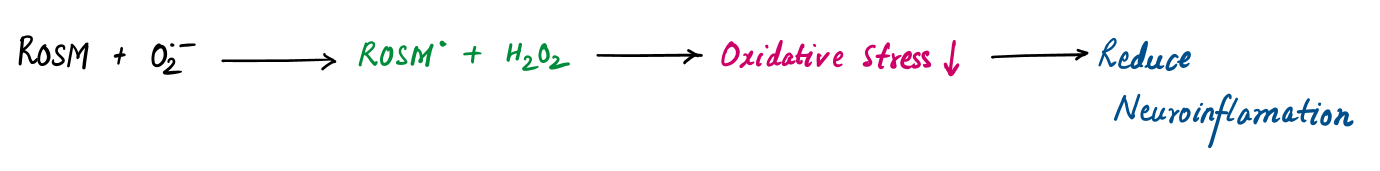
- Neuroprotective: Rosmarinic acid prevents neuronal damage by neutralizing ROS and reducing neuroinflammation, showing promise in neurodegenerative diseases like Alzheimer’s.
- Anti-microbial: It disrupts microbial membranes, inhibits biofilm formation, and blocks quorum sensing, combating pathogens such as E. coli and Candida albicans.
- Anti-microbial: It disrupts microbial membranes, inhibits biofilm formation, and blocks quorum sensing, combating pathogens such as E. coli and Candida albicans.
Conclusion : Analogy of Defensive-Offensive Military Strategy by Tulsi in term of ROS.
Reactive oxygen species (ROS) are the root cause of many diseases, including cancer, inflammation, and neurodegeneration. Tulsi’s bioactive compounds—ursolic acid, eugenol, and rosmarinic acid—work in harmony to combat ROS, employing a strategy to a defensive-offensive military tactic.
The concept of defensive-offensive strategy through diagram
Rosmarinic acid and eugenol serve as the defense, neutralizing excess ROS and safeguarding healthy cells, while ursolic acid takes the offensive role by increasing ROS selectively in cancer cells, leading to their targeted destruction. This coordinated action highlights Tulsi's ability to balance protection and precision.
Ursolic Acid (Offensive) | Increases ROS selectively in cancer cells, leading to mitochondrial dysfunction and apoptosis |
Rosmarinic Acid | Neutralizes ROS directly and inhibits their generation by chelating metal ions (e.g. Fe²⁺ ) |
Eugenol | Scavenges ROS and prevents lipid peroxidation, protecting cells from oxidative stress. |
d. Apigenin
Apigenin, a flavonoid in Tulsi (Ocimum sanctum), is a bioactive compound with diverse health benefits. It contributes to Tulsi's medicinal properties by targeting key biochemical pathways in the body.
Anti-diabetic, cardiovascular health and Skin health properties of Apigenin:
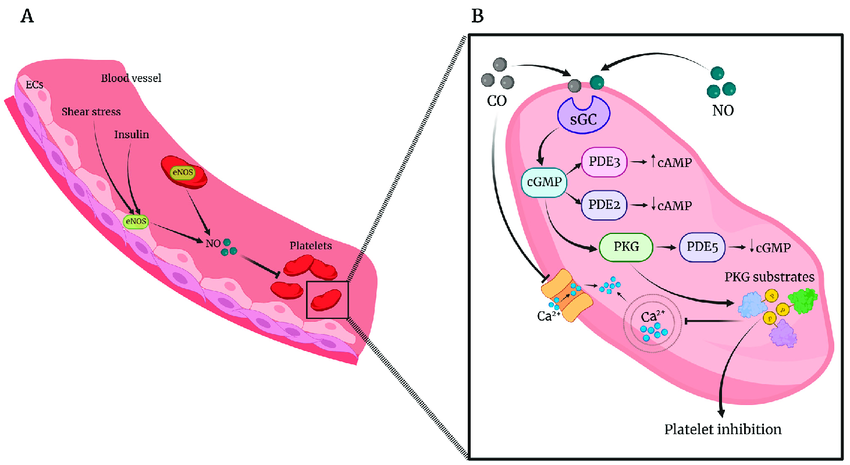
- Reduces Blood Pressure: Apigenin exhibits vasodilatory effects by increasing nitric oxide (NO) production. Nitric oxide (NO) is a vital molecule that maintains blood vessel health by promoting vasodilation, preventing clot formation (platelet aggregation), and reducing inflammation.
Apigenin enhances NO production by activating eNOS via the PI3K/Akt pathway, reducing oxidative stress, and supporting endothelial function (inner lining of blood vessel). This mechanism explaining Apigenin’s role in improving blood flow, lowering blood pressure, reducing hypertension or anxiety and protecting against cardiovascular diseases.
-
Anti-atherosclerotic Effects: It prevents the formation of plaques in arteries by reducing LDL oxidation and vascular inflammation.
- Regulates Glucose Metabolism: Apigenin enhances insulin sensitivity and reduces blood glucose levels by improving glucose uptake in cells.
- Protects Pancreatic Beta Cells: It reduces oxidative damage to pancreatic cells, preserving insulin production.
-
GABA Modulation: Apigenin acts on GABA-A receptors, promoting relaxation and reducing anxiety.
- Protects Neurons: Apigenin scavenges reactive oxygen species (ROS) in the brain, protecting neurons from oxidative damage.
3. Impacts of tulsi on air and environment (With Case-study):
a. Air Purification :
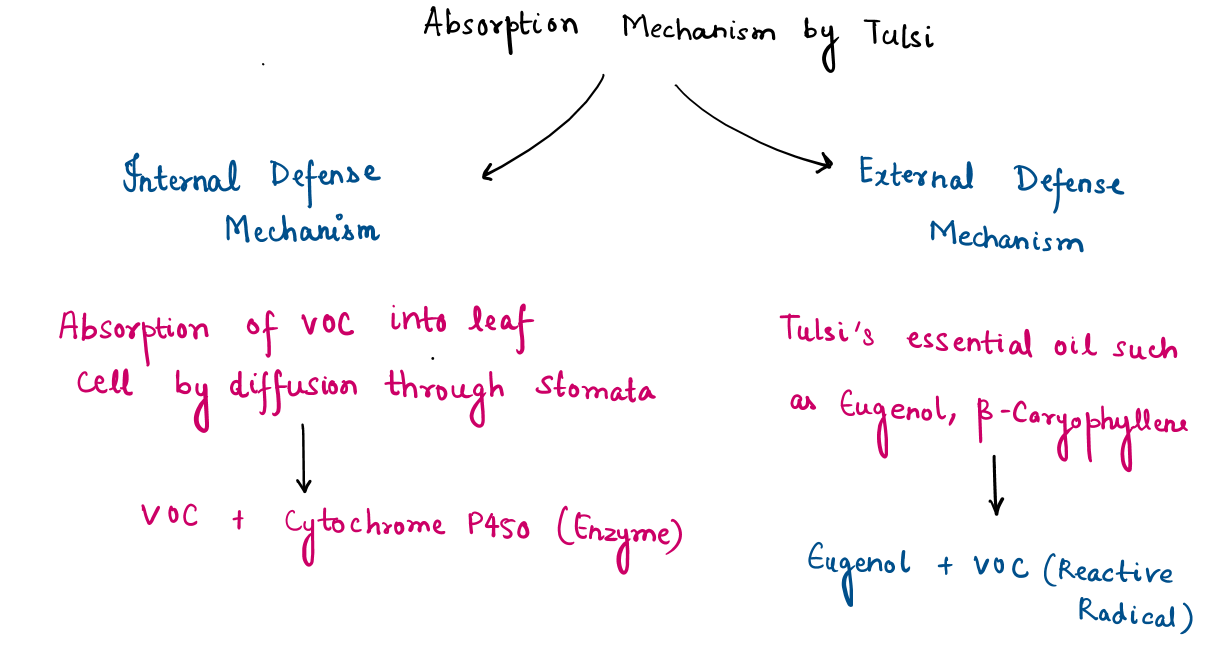
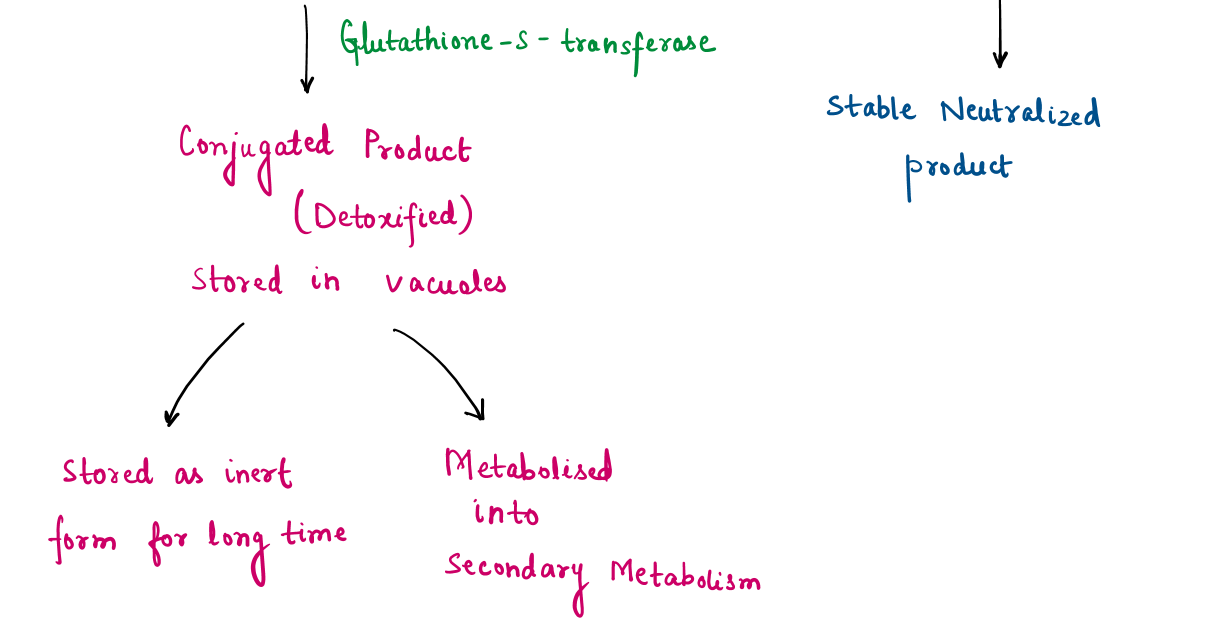
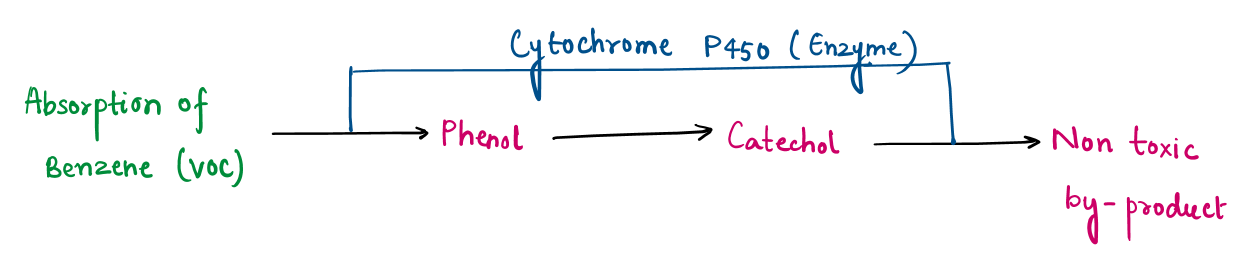
Mechanism of volatile organic compounds (VOCs) absorption:
Tulsi is well-known for its air-purifying abilities, including the absorption and detoxification of harmful Volatile Organic Compounds (VOCs) such as benzene, formaldehyde, and toluene. The process involves multiple biological and chemical pathways:
In addition to metabolizing VOCs through enzymatic pathways, Tulsi control air pollutants using various bioactive chemical of tulsi such as eugenol, methyl-eugenol, beta-caryophyllene, released into air. Eugenol interacts chemically with reactive VOCs, neutralizing them and preventing oxidative damage.
This dual mechanism : metabolic detoxification within the plant and chemical neutralization in the air help to enhances Tulsi’s effectiveness as a natural air purifier.
For example :
b. Climate regulation
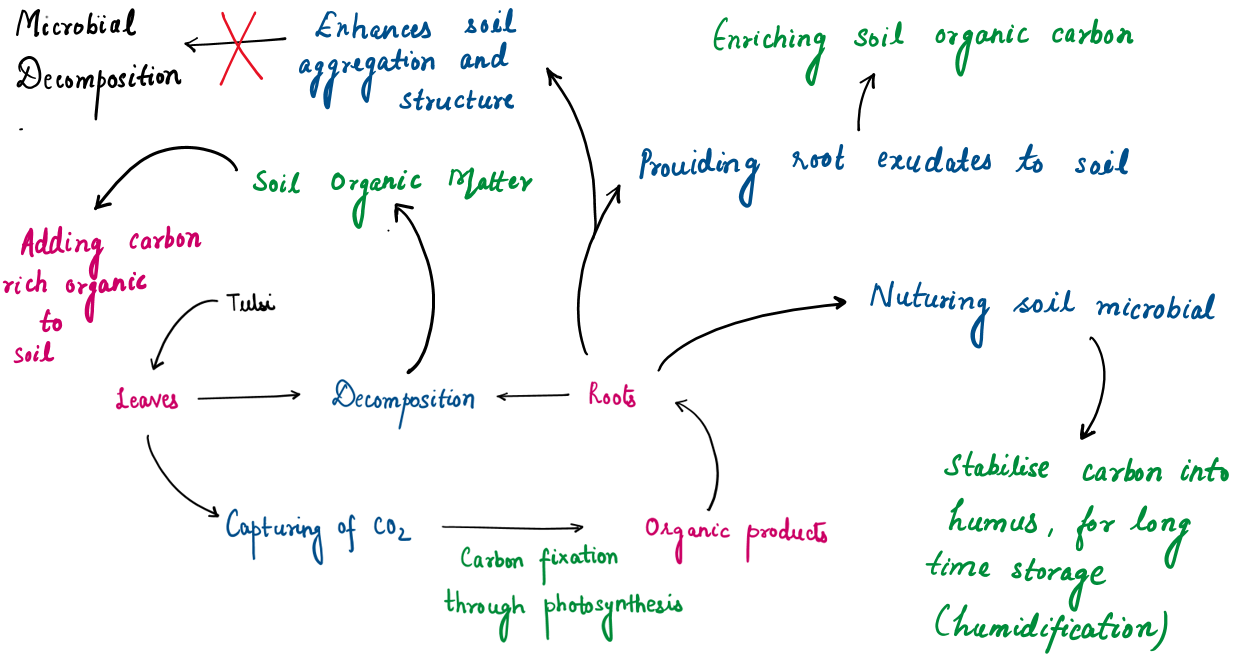
Carbon Sequestration: Role of Tulsi in Enhancing Soil Carbon Levels:
Carbon sequestration is the process of capturing and storing carbon dioxide (CO₂) from the atmosphere to fight climate change. Tulsi helps improve soil carbon levels in several simple ways:
Limitations: Tulsi’s smaller size means its sequestration capacity is limited compared to tall trees, but its medicinal and cultural significance makes it an important addition to sustainable urban and rural environments.
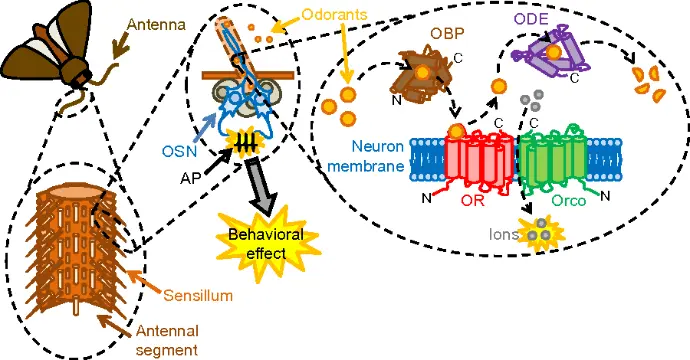
b. Insect Repellent Properties: Reaction Mechanism of Tulsi's Essential Oils in Repelling Pests
Tulsi, exhibit insect repellent properties due to their bioactive compounds, like eugenol, camphor, and thymol. These compounds interact with pests' sensory systems, disrupting their ability to locate hosts or survive in treated environments.
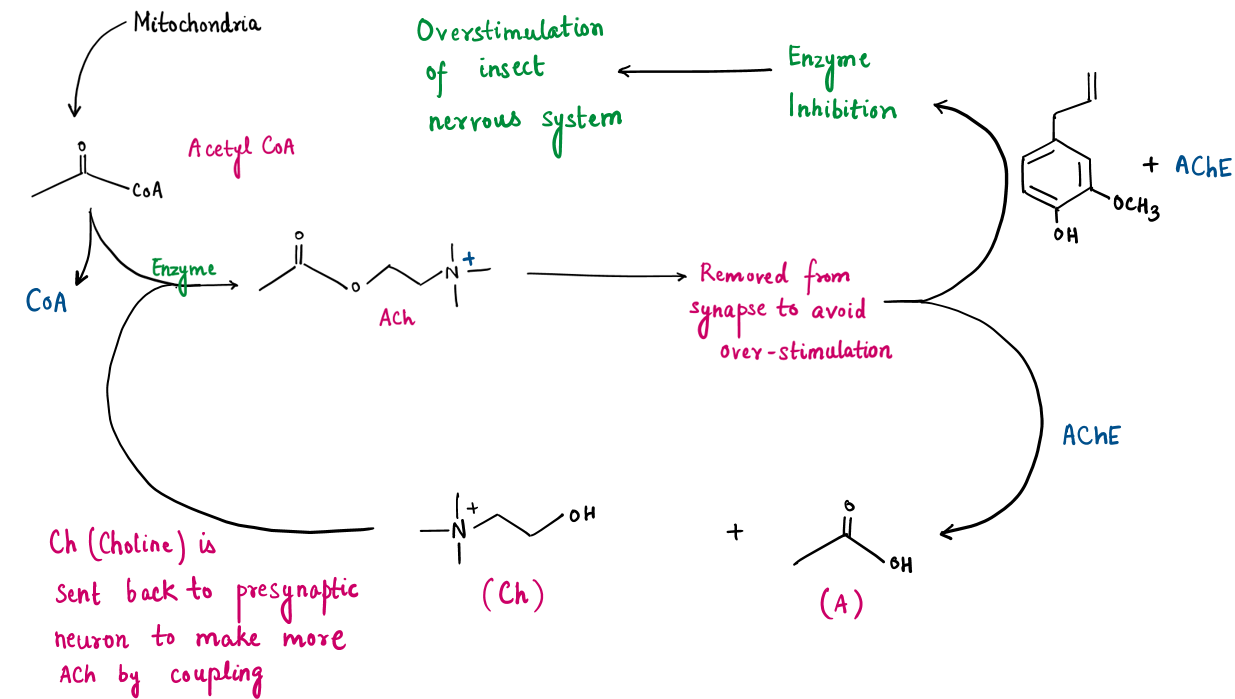
Neurotoxicity to Pests (Nervous System Disruption)
- Overstimulation of the insect nervous system.
- Paralysis and eventual death.
Disruption of Respiratory Function
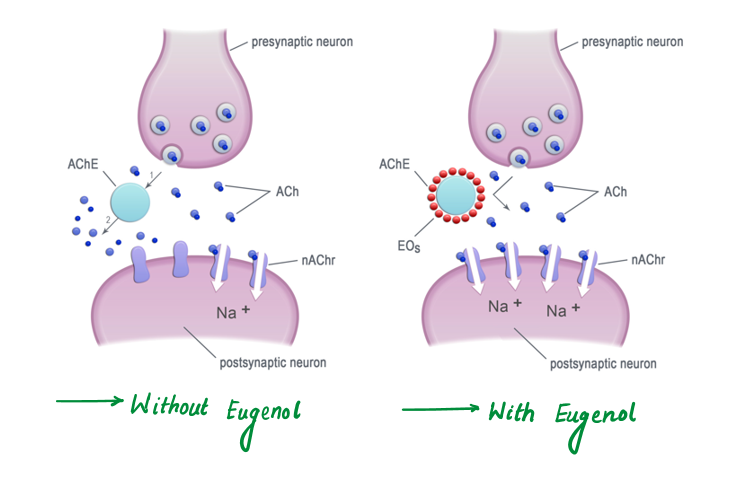
Compounds like eugenol act on acetylcholinesterase enzymes, inhibiting their function. This causes the accumulation of acetylcholine in the synapses, leading to:
Volatile compounds from essential oils such as camphor interfere with the insects’ spiracles (respiratory openings), making it difficult for them to breathe.
Anti-Feeding Properties (Gustatory Disruption)
Essential oil compounds act as anti-feedants, making plant surfaces unbearable to pests. When pests ingest treated plants, secondary metabolites like thymol disrupt their digestion or cause toxic effects.
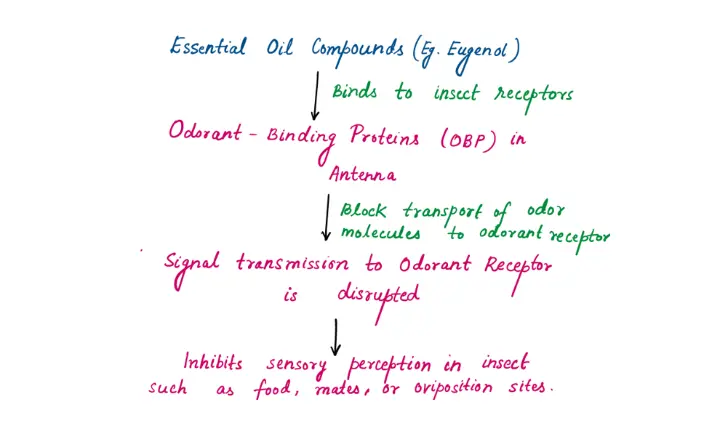
Interaction with Olfactory Receptors (Disruption of Smell)
Essential oil vapors contain volatile compounds (e.g., eugenol and camphor) that bind to odorant-binding proteins (OBPs) in insects' antennae in such a way :
Limitation
Although Tulsi contributes to climate change or air purification such as carbon sequestration, VOC-absorption, ozone protection and cooling, its scale of impact is much smaller compared to large-scale reforestation efforts or tall canopy trees. Its primary strength lies in localized air quality improvement and its medicinal and cultural significance.
4. Role of Tulsi in relieving different type of stress :
Tulsi (holy basil), is renowned for its adaptogenic properties, helping and protecting the body from various stressors. Here's how Tulsi helps alleviate different types of stress:
a. Toxicant stress (Heavy metal and chemical toxicity)
Tulsi has been widely studied for its ability to protect against toxicant stress caused by chemicals, heavy metals, and radiation. It prevents damage to vital organs like the liver, kidney, and brain by preventing genetic, immune, and cellular harm.
- Industrial Chemicals: Shields against toxic effects of compounds like butylparaben, carbon tetrachloride, copper sulfate, and ethanol.
- Pesticides: Mitigates the damage caused by pesticides such as rogor, chlorpyrifos, endosulfan, and lindane.
- Pharmaceutical Drugs: Reduces toxicity from drugs like acetaminophen, paracetamol, haloperidol, and anti-tubercular medications.
- Heavy Metals: Protects against lead, arsenic, cadmium, chromium, and mercury by reducing oxidative stress.
- Radiation: Acts as a radio-protective agent by scavenging free radicals and preventing oxidative cellular and chromosomal damage, thereby reducing organ injury and enhancing survival after radiation exposure.
b. Physical Stress
Tulsi is equally effective in alleviating the harmful effects of physical stress caused by prolonged exertion, physical restraint, exposure to cold, and excessive noise. These stressors disrupt homeostasis and can damage biochemical pathways, organ function, and overall health.
- Oxidative Stress Reduction: Tulsi reduces oxidative tissue damage caused by physical stressors, helping to protect vital organs.
- Normalization of Biochemical Parameters: It restores physiological and biochemical balance disrupted by physical stress.
- Noise Stress Mitigation: Experimental studies have shown that Tulsi reduces acute and chronic noise-induced stress, improving brain neurotransmitter levels, immune responses, and cardiovascular function.
c. Metabolic Stress
Metabolic stress, occur due to poor diet, low physical activity, and psychological stress, is a consequence of modern lifestyles and contributes to metabolic syndrome. This condition, also called as "prediabetes", includes obesity, hypertension, high cholesterol, and poor glucose regulation, leading to chronic inflammation and increased risks of diabetes, heart disease, and stroke.
- Anti-Diabetic Properties: Tulsi reduces blood glucose levels, improves insulin sensitivity, and protects pancreatic cells from oxidative damage.
- Lipid Profile Improvement: Tulsi lowers LDL cholesterol, triglycerides, and overall lipid synthesis, while promoting healthy liver function and bile acid production.
- Weight Management: Prevents weight gain and related complications by regulating metabolism.
- Cardiovascular Protection: Protects blood vessels and organs from damage caused by atherosclerosis and high-fat diets.
- Anti-Inflammatory Effects: Reduces both acute and chronic inflammation by inhibiting cyclooxygenase and lipoxygenase pathways, comparable to anti-inflammatory drugs like aspirin and ibuprofen.
d. Mental stress : Enhancing cognitive function
Modern lifestyles are often associated with high levels of psychological stress due to fast-paced living, constant demands, and exposure to toxic chemicals. Tulsi helps reduce mental stress through its unique psycho-therapeutic properties.
- Anti-Anxiety and Anti-Depressant Effects: Studies reveal that Tulsi’s effects are comparable to drugs like diazepam, reducing anxiety, depression, and stress-related symptoms.
- Memory and Cognitive Benefits: Tulsi enhances memory, cognitive function, and protects against age-related memory deficits.
- Stress Reduction in Humans: Clinical trials report that Tulsi improves general stress scores, alleviates lack of sleep and sexual problems, and reduces exhaustion.
e. Infective stress
Tulsi exhibits broad-spectrum antimicrobial properties, offering protection against bacterial, viral, and fungal infections. It strengthens the immune system and supports recovery from various infectious diseases.
- Anti-Bacterial Action: Tulsi shows activity against bacterial infections, including urinary tract infections, typhoid, cholera, tuberculosis, and acne. Its effectiveness also extends to food- and water-borne pathogens, supporting its use in food preservation and water purification.
- Anti-Viral Action: Experimental evidence suggests that Tulsi helps manage viral infections like mosquito-borne diseases such as dengue, malaria, and filariasis.
- Anti-Fungal Action: Tulsi combats fungal infections, making it effective in treating skin, wound, and systemic fungal conditions.
- Oral Health: Tulsi’s activity against the bacteria responsible for tooth decay, highlights its use as a herbal mouthwash for treating bad breath, gum disease, and mouth ulcers. Clinical trials confirm that Tulsi mouthwash is as effective as commercial products like chlorhexidine.
5. Sustainable Farming and Nurturing of Tulsi
The cultivation of Tulsi not only provides economic opportunities for rural communities but also contributes to environmental conservation and global health.
Tulsi works best when grown organically in rural areas free from environmental pollution. Studies indicate that plants cultivated in polluted regions tend to accumulate higher levels of toxic elements, reducing their medicinal efficiency.
Tulsi cultivation improves soil fertility by contributing organic matter through its biomass. It also prevents soil erosion and enhances biodiversity in agricultural ecosystems. Intercropping Tulsi with other crops can promote ecological balance and improve overall yield without the need for chemical inputs.
As naturally pest-resistant, tulsi plant is not prone to serious pest attack. So, there is no or minimal need of chemical for protection against some common pest like leaf hopper.
While Tulsi is resilient and hardy enough to grow on any kind of soil in tropical and sub-tropical climates , it is sensitive to frost and extreme cold. Sustainable farming practices include adapting to seasonal changes by providing protection during colder months or growing Tulsi in controlled environments otherwise it will produce low content of essential oil.
Organizations like Organic India Pvt Ltd have established sustainable business models that empower rural farmers through fair trade practices. These initiatives provide farmers with a dignified livelihood while promoting ecological integrity.
Ethical farming methods ensure the use of non-GMO seeds (Non-Genetically modified seed), sustainable harvesting practices, and minimal environmental impact.

By supporting small-scale Tulsi farmers, ethical companies promote rural development, food security, and environmental sustainability, making Tulsi farming a model for ecological harmony.
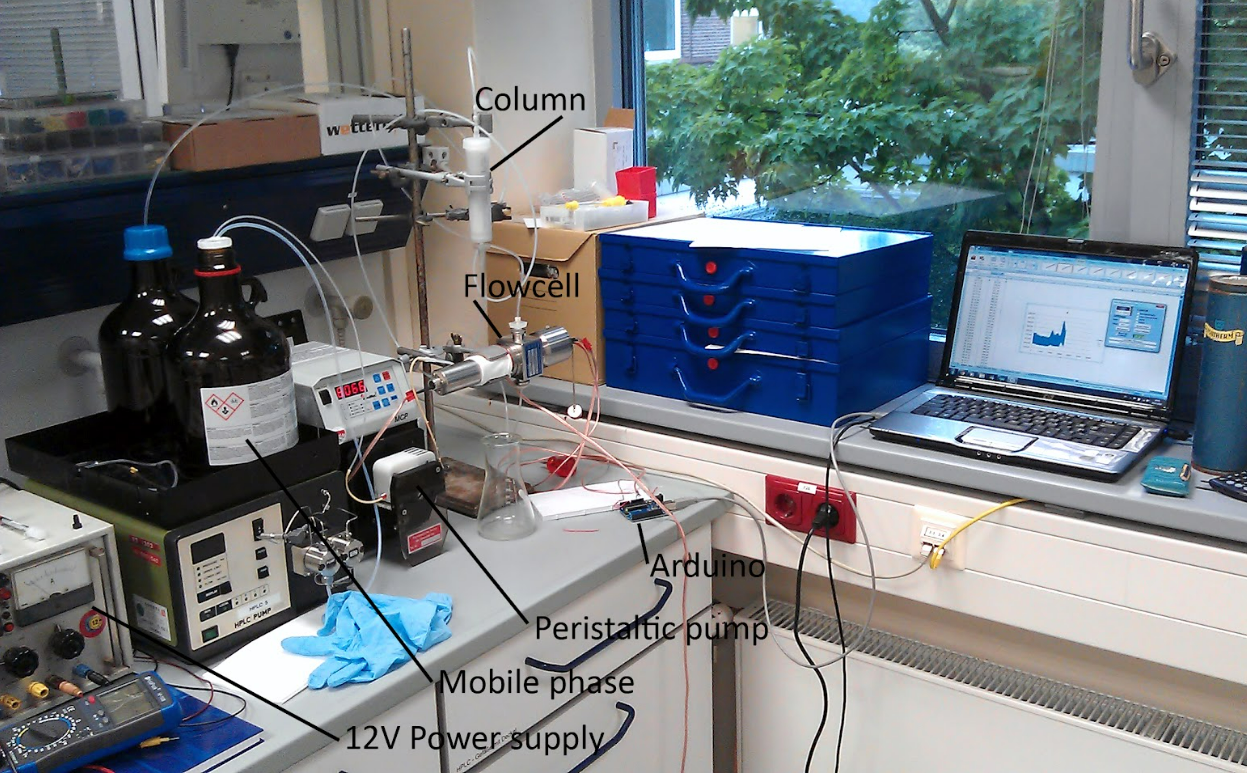
To preserve its medicinal value, strongest quality standards are necessary at every stage of cultivation, harvesting, and processing. Techniques like high-performance liquid chromatography and microscopic assays help ensure the botanical integrity of Tulsi products.
HLPC pump device
Regular harvesting of leaves is done carefully to encourage continuous growth without harming the plant, maintaining both yield and quality. There is a need to the shift from industrialized monoculture farming (Green-Revolution) to localized, organic, and sustainable farming practices.
The Growing Economic Impact of Ayurveda: Tulsi as a Catalyst
As Ayurveda's contribution to India's GDP has grown from 4% in 2004 to an impressive 25% in 2024, initiatives like Tulsi cultivation symbolize the successful fusion of tradition and modern economic growth, showcasing the global potential of Indian wisdom.
Projected Growth of Ayurveda Market in India (2019-2028)
Sources :
- In 2019, the Ayurveda market in India was valued at ₹335 billion, with projections to exceed ₹1 trillion by 2025.
- In 2021, the market was worth ₹515.5 billion (approximately USD 6.6 billion), expected to grow at a CAGR of 19.8%.
- By 2023, the industry reached ₹574.5 billion, with projections to hit ₹1.2 trillion (USD 16.27 billion) by FY28.
- The export value of Ayurvedic and herbal products from India was approximately USD 651 million in FY2024.
Over the past decade, the Ayurveda industry has witnessed exponential growth. With the market projected to surpass ₹1 trillion by 2025 and exports exceeding $650 million in 2024, Ayurveda has emerged as a vital contributor to India’s economic growth, global health, and sustainability goals.
6. Connection to Nature: Tulsi as Aromatherapy
Tulsi bridges the gap between humans and nature, offering profound health and emotional benefits:
Connecting with nature is an effective way to heal mind and body
- Healing Influence: Tulsi connects households to the natural world, countering stress and sedentary lifestyles.
- Air Purification and Calming Effects: Tulsi’s VOCs purify the air and reduce anxiety, improving mental health.
- Global Relevance: Aligns with efforts to reduce “nature deficit” and promotes well-being through a closer bond with nature.
By embodying the power of nature, Tulsi fosters a healthier, balanced, and connected lifestyle.
Tulsi Gowda - Encyclopedia of Forest (Indian Environmentalist, honored with Padma Shri 2020)
Conclusion :
Tulsi: A Symbol of Health and Environmental Well-Being
Tulsi, often called the "Queen of Herbs," holds a special place in both traditional culture and modern science. It is not just a sacred plant but also a natural healer. Tulsi’s compounds help reduce stress, fight infections, and improve mental and physical health. It works as an air purifier by releasing compounds that clean the air and calm the mind. Additionally, it plays a key role in environmental well-being by improving soil quality, supporting biodiversity, and promoting sustainability. Tulsi reminds us how closely human health is tied to the environment.
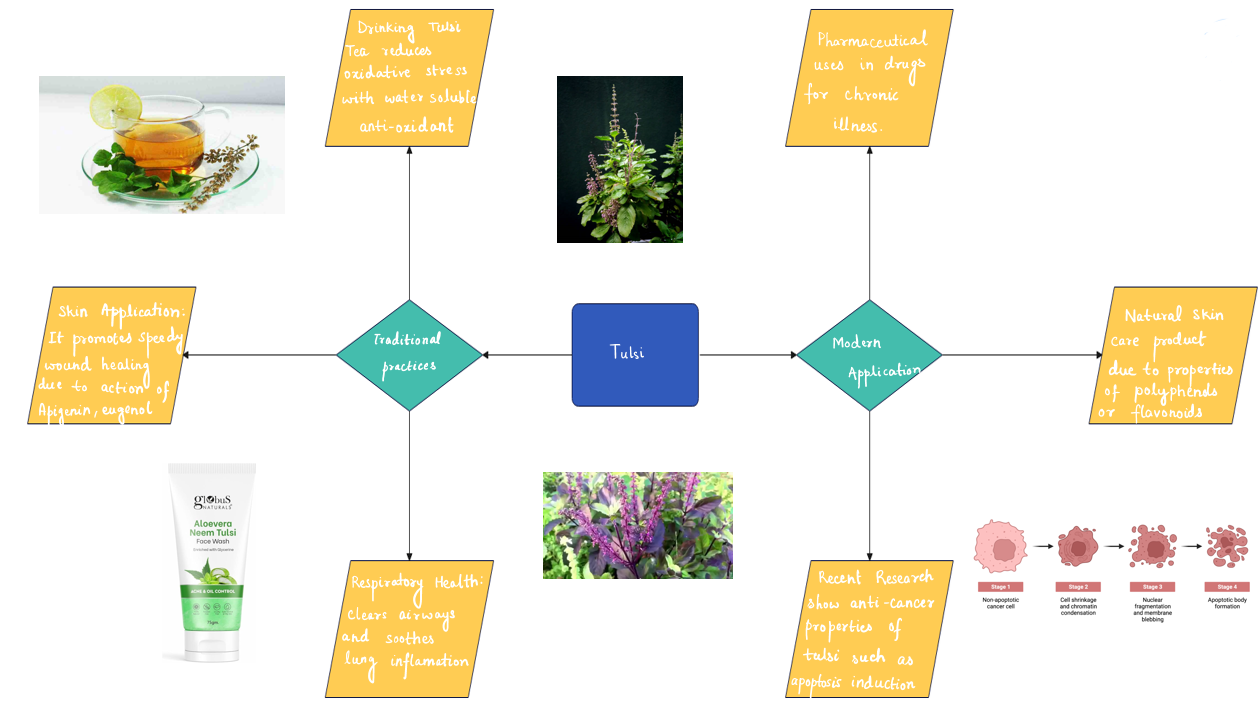
Modern application of Tulsi Biochemistry and their traditional practices
Tulsi Pujan Diwas: Honoring Nature and Science
Celebrating Tulsi Pujan Diwas is not just about tradition—it’s a way of reconnecting with nature and appreciating its benefits. This day inspires people to respect plants like Tulsi for their ability to heal and protect. It also shows how ancient traditions align with modern science, addressing today’s challenges like pollution, climate change, and stress-filled lifestyles. By bridging tradition and innovation, Tulsi Pujan Diwas encourages us to adopt sustainable practices and foster a deeper connection to the natural world.
Reference :
1. Paidi, R. K., Jana, M., Raha, S., McKay, M., Sheinin, M., Mishra, R. K., & Pahan, K. (2021). Eugenol, a Component of Holy Basil (Tulsi) and Common Spice Clove, Inhibits the Interaction Between SARS-CoV-2 Spike S1 and ACE2 to Induce Therapeutic Responses. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology, 16(4), 743–755. https://doi.org/10.1007/s11481-021-10028-1
2. Wang, G. R., Zhu, Y., Halushka, P. V., Lincoln, T. M., & Mendelsohn, M. E. (1998). Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America, 95(9), 4888–4893. https://doi.org/10.1073/pnas.95.9.4888
3. Nagababu, E., & Lakshmaiah, N. (1992). Inhibitory effect of eugenol on non-enzymatic lipid peroxidation in rat liver mitochondria. Biochemical pharmacology, 43(11), 2393–2400. https://doi.org/10.1016/0006-2952(92)90318-d
4. Wikipedia contributors. (2024, November 22). Eugenol. In Wikipedia, The Free Encyclopedia. Retrieved 11:19, December 11, 2024, from https://en.wikipedia.org/w/index.php?title=Eugenol&oldid=1258908707
5. Kornel, A., Nadile, M., Retsidou, M. I., Sakellakis, M., Gioti, K., Beloukas, A., Sze, N. S. K., Klentrou, P., & Tsiani, E. (2023). Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. International journal of molecular sciences, 24(8), 7414. https://doi.org/10.3390/ijms24087414
6. Khwaza, V., Oyedeji, O. O., & Aderibigbe, B. A. (2020). Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. International journal of molecular sciences, 21(16), 5920. https://doi.org/10.3390/ijms21165920
7. Panda, S. S., Thangaraju, M., & Lokeshwar, B. L. (2022). Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules, 27(24), 8981. https://doi.org/10.3390/molecules27248981
8. Li, Xinyuan & Fang, Pu & Mai, Jietang & Choi, Eric & Wang, Hong & Yang, Xiaofeng. (2013). Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Journal of hematology & oncology. 6. 19. 10.1186/1756-8722-6-19.
9. Salma Akter, Jingjing Huang, Cezary Waszczak, Silke Jacques, Kris Gevaert, Frank Van Breusegem, Joris Messens, Cysteines under ROS attack in plants: a proteomics view, Journal of Experimental Botany, Volume 66, Issue 10, May 2015, Pages 2935–2944, https://doi.org/10.1093/jxb/erv044
10. Wikipedia contributors. (2024, October 12). Ursolic acid. In Wikipedia, The Free Encyclopedia. Retrieved 13:06, December 14, 2024, from https://en.wikipedia.org/w/index.php?title=Ursolic_acid&oldid=1250847983
11. Rosmarinic acid and its derivatives: Current insights on anticancer potential and other biomedical applications (2023) Biomedicine & Pharmacotherapy. https://doi.org/10.1016/j.biopha.2023.114687 (Accessed: 14 December 2024).12. Roman Pavela et al. (2023) Biomass yield potential of Tulsi (ocimum sanctum L.) in European conditions, Industrial Crops and Products.
https://doi.org/10.1016/j.indcrop.2023.116365 (Accessed: 15 December 2024).
13. Wikipedia contributors. (2024, September 10). Rosmarinic acid. In Wikipedia, The Free Encyclopedia. Retrieved 14:07, December 12, 2024, from https://en.wikipedia.org/w/index.php?title=Rosmarinic_acid&oldid=1245077692
14. Iza F. Pérez-Ramírez et al. (2017) Effect of ocimum sanctum and Crataegus pubescens aqueous extracts on obesity, inflammation, and glucose metabolism, Journal of Functional Foods. https://doi.org/10.1016/j.jff.2017.05.028 (Accessed: 15 December 2024).
15. Wikipedia contributors. (2024, October 25). Apigenin. In Wikipedia, The Free Encyclopedia. Retrieved 14:26, December 15, 2024, from https://en.wikipedia.org/w/index.php?title=Apigenin&oldid=1253427149
16. Tangpao, T. et al. (2022) Volatile organic compounds from basil essential oils: Plant taxonomy, biological activities, and their applications in Tropical Fruit Productions, MDPI. Available at: https://www.mdpi.com/2311-7524/8/2/144 (Accessed: 16 December 2024).
17. Lopamudra Sethi and Preetha Bhadra (2020) (PDF) a review paper on Tulsi plant (ocimum sanctum L.), Indian Journal of Natural Sciences (Accessed: 18 December 2024).
18. Singh, N., Hoette, Y., & Miller, D. R. (2002). Tulsi: The mother medicine of nature. International Institute of Herbal Medicine19. Wangcharoen, W., & Morasuk, W. (2007). Antioxidant capacity and phenolic content of holy basil. Songklanakarin J Sci Technol, 29(5), 1407-1415.
20. Panda, V. S., & Naik, S. R. (2009). Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents. Alternative Medicine Review, 14(2), 161.
21. Manikandan, P., Vidjaya Letchoumy, P., Prathiba, D., & Nagini, S. (2008). Combinatorial chemopreventive effect of Azadirachta indica and Ocimum sanctum on oxidant-antioxidant status, cell proliferation, apoptosis and angiogenesis in a rat forestomach carcinogenesis model. Singapore medical journal, 49(10), 814.
22. Shah, K. O. M. A. L., & Verma, R. J. (2012). Protection against butyl p-hydroxybenzoic acid induced oxidative stress by Ocimum sanctum extract in mice liver. Acta Pol Pharm, 69(5), 865-70.
23. Enayatallah, S. A. M., Shah, S. N., & Bodhankar, S. L. K. (2004). A study of hepatoprotective activity of Ocimum sanctum (Krishna tulas) extracts in chemically induced liver damage in albino mice. JOURNAL OF ECOPHYSIOLOGY AND OCCUPATIONAL HEALTH, 4, 89-96.
24. SHYAMALA, A. C., & DEVAKI, T. (1996). Studies on peroxidation in rats ingesting copper sulphate and effect of subsequent treatment with Ocimum sanctum. Journal of clinical biochemistry and nutrition, 20(2), 113-119.
25. Bawankule, D. U., Pal, A., Gupta, S., Yadav, S., Misra, A., Rastogi, S., ... & Khanuja, S. P. (2008). Protective effect of Ocimum sanctum on ethanol-induced oxidative stress in Swiss Albino Mice brain. Toxicol Int, 5, 121-5.
26. Makwana, M., & Rathore, H. S. (2011). Prevention of hepatorenal toxicity of acetaminophen with Ocimum sanctum in mice. Int J Pharm Technol, 3, 1385-96.
27. Mahaprabhu, R., Bhandarkar, A. G., Jangir, B. L., Rahangadale, S. P., & Kurkure, N. V. (2011). Ameliorative effect of Ocimum Sanctum on meloxicam induced toxicity in wistar rats. Toxicology International, 18(2), 130.
28. Lahon, K., & Das, S. (2011). Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharmacognosy research, 3(1), 13.
29. Pemminati, S., Nair, V., Dorababu, P., Gopalakrishna, H. N., & Pai, M. R. S. M. (2007). Effect of ethanolic leaf extract of Ocimum sanctum on haloperidol-induced catalepsy in albino mice. Indian Journal of pharmacology, 39(2), 87-89.
30. Ubaid, R. S., Anantrao, K. M., Jaju, J. B., & Mateenuddin, M. D. (2003). Effect of Ocimum sanctum (OS) leaf extract on hepatotoxicity induced by antitubercular drugs in rats. Indian journal of physiology and pharmacology, 47(4), 465-470.
31. Karamala, S. K., Srilatha, C. H., Anjaneyulu, Y., ChandraSekharaRao, T. S., Sreenivasulu, D., & Pidugu, A. P. (2011). Hematobiochemical changes of lead poisoning and amelioration with Ocimum sanctum in wistar albino rats. Veterinary world, 4(6), 260.
32. Banu, G. S., Kumar, G., & Murugesan, A. G. (2009). RETRACTED: Effects of leaves extract of Ocimum sanctum L. on arsenic-induced toxicity in Wistar albino rats.
33. Sharma, M. K., Kumar, M., & Kumar, A. (2002). Ocimum sanctum aqueous leaf extract provides protection against mercury induced toxicity in Swiss albino mice.
34. Reshma, K., Kamalaksh, S., Bindu, Y. S., Pramod, K., Asfa, A., Amritha, D., ... & Chandrashekar, R. (2012). Tulasi (Ocimum Sanctum) as radioprotector in head and neck cancer patients undergoing radiation therapy. Biomedicine, 32(1), 39-44.
35. Singh, N., Verma, P., Pandey, B. R., & Bhalla, M. (2012). Therapeutic potential of Ocimum sanctum in prevention and treatment of cancer and exposure to radiation: An overview. International Journal of pharmaceutical sciences and drug research, 4(2), 97-104.
36. Uma Devi, P., Ganasoundari, A., Vrinda, B., Srinivasan, K. K., & Unnikrishnan, M. K. (2000). Radiation protection by the ocimum flavonoids orientin and vicenin: mechanisms of action. Radiation research, 154(4), 455-460.
37. Reshma, K., Rao, A. V., Dinesh, M., & Vasudevan, D. M. (2008). Radioprotective effects of ocimum flavonoids on leukocyte oxidants and antioxidants in oral cancer. Indian Journal of Clinical Biochemistry, 23, 171-175.
38. Samson, J., Sheeladevi, R., & Ravindran, R. (2007). Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology, 28(3), 679-685.
39. Archana, R., & Namasivayam, A. (2002). A comparative study of different crude extracts of Ocimum sanctum on noise stress. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 16(6), 579-580.
40. Sembulingam, K., Sembulingam, P., & Namasivayam, A. (1999). Effect of ocimum sanctum linn on changes in leucocytes of albino rats induced by acute noise stress. Indian journal of physiology and pharmacology, 43(1), 137-140.
41. Sembulingam, K., Sembulingam, P., & Namasivayam, A. (2005). Effect of Ocimum sanctum Linn on the changes in central cholinergic system induced by acute noise stress. Journal of ethnopharmacology, 96(3), 477-482.
42. Ahmad, M. Z., Ali, M., & Mir, S. R. (2012). Anti-diabetic activity of Ocimum sanctum L. roots and isolation of new phytoconstituents using two-dimensional nuclear magnetic resonance spectroscopy. J Pharmacogn Phytother, 4, 75-85.
43. Singh, P. K., Baxi, D., Banerjee, S., & Ramachandran, A. V. (2012). Therapy with methanolic extract of Pterocarpus marsupium Roxb and Ocimum sanctum Linn reverses dyslipidemia and oxidative stress in alloxan induced type I diabetic rat model. Experimental and Toxicologic Pathology, 64(5), 441-448.
44. Suanarunsawat, T., & Songsak, T. (2005). Anti-hyperglycaemic and anti-dyslipidaemic effect of dietary supplement of white Ocimum Sanctum Linnean before and after STZ-induced diabetes mellitus. International Journal of Diabetes and Metabolism, 13(1), 18-23.
45. Suanarunsawat, T., Devakul Na Ayutthaya, W., Songsak, T., Thirawarapan, S., & Poungshompoo, S. (2011). Lipid‐lowering and antioxidative activities of aqueous extracts of Ocimum sanctum L. leaves in rats fed with a high‐cholesterol diet. Oxidative Medicine and Cellular Longevity, 2011(1), 962025.
46. Dahiya, K., Sethi, J., Dhankhar, R., Singh, V., Singh, S. B., Yadav, M., ... & Sachdeva, A. (2011). Effect of Ocimum sanctum on homocysteine levels and lipid profile in healthy rabbits. Archives of physiology and biochemistry, 117(1), 8-11.
47. Samak, G., Rao, M. S., Kedlaya, R., & Vasudevan, D. M. (2007). Hypolipidemic efficacy of Ocimum sanctum in the prevention of atherogenesis in male albino rabbits. Pharmacologyonline, 2, 115-27.
48. Agrawal, P., Rai, V., & Singh, R. B. (1996). Randomized placebo-controlled, single blind trial of holy basil leaves in patients with noninsulin-dependent diabetes mellitus. International journal of clinical pharmacology and therapeutics, 34(9), 406-409.
49. RAI MSC, UV MANI MSC PHD FICN AND UM IYER MSC PHD, V. (1997). Effect of Ocimum sanctum leaf powder on blood lipoproteins, glycated proteins and total amino acids in patients with non-insulin-dependent diabetes mellitus. Journal of nutritional & environmental medicine, 7(2), 113-118.
50. Devra, D. K., Mathur, K. C., Agrawal, R. P., Bhadu, I., Goyal, S., & Agarwal, V. (2012). Effect of Tulsi (Ocimum sanctum Linn.) on clinical and biochemical parameters of metabolic syndrome. Journal of Natural Remedies, 63-67.
51. Hannan, J. M. A., Marenah, L., Ali, L., Rokeya, B., Flatt, P. R., & Abdel-Wahab, Y. H. A. (2006). Ocimum sanctum leaf extracts stimulate insulin secretion from perfused pancreas, isolated islets and clonal pancreatic β-cells. Journal of Endocrinology, 189(1), 127-136.
52. Chattopadhyay RR. Hypoglycemic effect of Ocimum sanctum leaf extract in normal and streptozotocin diabetic rats. Indian J Exp Biol. 1993;31
53. Gholap, S., & Kar, A. (2004). Hypoglycaemic effects of some plant extracts are possibly mediated through inhibition in corticosteroid concentration. Die Pharmazie, 59(11), 876–878.
54. Singh, S., & Majumdar, D. K. (1997). Evaluation of antiinflammatory activity of fatty acids of Ocimum sanctum fixed oil. Indian journal of experimental biology, 35(4), 380–383.
55. Singh S. (1998). Comparative evaluation of antiinflammatory potential of fixed oil of different species of Ocimum and its possible mechanism of action. Indian journal of experimental biology, 36(10), 1028–1031.
56. Singh S, Majumdar DK. Anti-inflammatory and antipyretic activities of Ocimum sanctum fixed oil. Int Pharmacogn. 1995;33:288–92
57. Kelm, M. A., Nair, M. G., Strasburg, G. M., & DeWitt, D. L. (2000). Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine : international journal of phytotherapy and phytopharmacology, 7(1), 7–13. https://doi.org/10.1016/S0944-7113(00)80015-X
58. Chatterjee, M., Verma, P., Maurya, R., & Palit, G. (2011). Evaluation of ethanol leaf extract of Ocimum sanctum in experimental models of anxiety and depression. Pharmaceutical biology, 49(5), 477–483. https://doi.org/10.3109/13880209.2010.523832
59. Tabassum, I., Siddiqui, Z. N., & Rizvi, S. J. (2010). Effects of Ocimum sanctum and Camellia sinensis on stress-induced anxiety and depression in male albino Rattus norvegicus. Indian journal of pharmacology, 42(5), 283–288.
https://doi.org/10.4103/0253-7613.70108
60. Raghavendra, M., Maiti, R., Kumar, S., & Acharya, S. (2013). Role of aqueous extract of Azadirachta indica leaves in an experimental model of Alzheimer's disease in rats. International journal of applied & basic medical research, 3(1), 37–47. https://doi.org/10.4103/2229-516X.112239
61. Pemminati S, Gopalakrishna HN, Venkatesh V, Rai A, Shetty S, Vinod A, et al. Anxiolytic effect of acute administration of ursolic acid in rats. Res J Pharm Biol Chem Sci. 201162. Moinuddin G, Devi K, Satish H, Khajuria DK. Comparative pharmacological evaluation of Ocimum sanctum and imipramine for antidepressant activity. Lat Am J Pharm. 2011
63. Giridharan, V. V., Thandavarayan, R. A., Mani, V., Ashok Dundapa, T., Watanabe, K., & Konishi, T. (2011). Ocimum sanctum Linn. leaf extracts inhibit acetylcholinesterase and improve cognition in rats with experimentally induced dementia. Journal of medicinal food, 14(9), 912–919. https://doi.org/10.1089/jmf.2010.1516
64. Dokania M, Kishore K, Sharma PK. Effect of Ocimum sanctum extract on sodium nitrite-induced experimental amnesia in mice. Thai J Pharma Sci. 2011
65. Bhattacharyya, D., Sur, T. K., Jana, U., & Debnath, P. K. (2008). Controlled programmed trial of Ocimum sanctum leaf on generalized anxiety disorders. Nepal Medical College journal : NMCJ, 10(3), 176–179.
66. Saxena, R. C., Singh, R., Kumar, P., Negi, M. P., Saxena, V. S., Geetharani, P., Allan, J. J., & Venkateshwarlu, K. (2012). Efficacy of an Extract of Ocimum tenuiflorum (OciBest) in the Management of General Stress: A Double-Blind, Placebo-Controlled Study. Evidence-based complementary and alternative medicine : eCAM, 2012, 894509. https://doi.org/10.1155/2012/894509
67. Vasudevan P, Kashyap S, Sharma S. Bioactive botanicals from basil (Ocimum sp.) J Sci Ind Res (C) 1999.
68. Mediratta, P. K., Sharma, K. K., & Singh, S. (2002). Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. Journal of ethnopharmacology, 80(1), 15–20. https://doi.org/10.1016/s0378-8741(01)00373-7
69. Mondal, S., Varma, S., Bamola, V. D., Naik, S. N., Mirdha, B. R., Padhi, M. M., Mehta, N., & Mahapatra, S. C. (2011). Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. Journal of ethnopharmacology, 136(3), 452–456. https://doi.org/10.1016/j.jep.2011.05.012
70. malatha R, Babu KN, Karthik M, Ramesh R, Kumar BD, Kumar PU. Immunomodulatory activity and Th1/Th2 cytokine response of Ocimum sanctum in myelosuppressed swiss albino mice. Trends Med Res. 2011
71. Ali H, Dixit S. In vitro antimicrobial activity of flavanoids of Ocimum sanctum with synergistic effect of their combined form. Asian Pac J Trop Dis. 2012;2:S396–8
72. Singh, S., Malhotra, M., & Majumdar, D. K. (2005). Antibacterial activity of Ocimum sanctum L. fixed oil. Indian journal of experimental biology, 43(9), 835–837.
73. Shokeen, P., Ray, K., Bala, M., & Tandon, V. (2005). Preliminary studies on activity of Ocimum sanctum, Drynaria quercifolia, and Annona squamosa against Neisseria gonorrhoeae. Sexually transmitted diseases, 32(2), 106–111. https://doi.org/10.1097/01.olq.0000152821.23777.90
74. Mandal, S., Mandal, M. D., & Pal, N. K. (2012). Enhancing chloramphenicol and trimethoprim in vitro activity by Ocimum sanctum Linn. (Lamiaceae) leaf extract against Salmonella enterica serovar Typhi. Asian Pacific journal of tropical medicine, 5(3), 220–224. https://doi.org/10.1016/S1995-7645(12)60028-5
75. Parag S, Vijyayshree N, Rami B, Patil B. Antibacterial activity of Ocimum sanctum Linn. and its application in water purification. Res J Chem Environ. 2010;14:46–50.
76. Farivar TN, Fard AH, Zahedani SS, Naderi M, Moud BS. Anti tuberculosis effect of Ocimum sanctum extracts in in vitro and macrophage culture. J Med Sci. 2006.
77. Balakumar, S., Rajan, S., Thirunalasundari, T., & Jeeva, S. (2011). Antifungal activity of Ocimum sanctum Linn. (Lamiaceae) on clinically isolated dermatophytic fungi. Asian Pacific journal of tropical medicine, 4(8), 654–657. https://doi.org/10.1016/S1995-7645(11)60166-1
78. Chandra R, Dwivedi V, Shivam K, Jha AK. Detection of antimicrobial activity of Oscimum sanctum (Tulsi) and trigonella foenum graecum (Methi) against some selected bacterial and fungal strains. Res J Pharm Biol Chem Sci. 2011;2:809–13
79. Rajamma, A. J., Dubey, S., Sateesha, S. B., Tiwari, S. N., & Ghosh, S. K. (2011). Comparative larvicidal activity of different species of Ocimum against Culex quinquefasciatus. Natural product research, 25(20), 1916–1922. https://doi.org/10.1080/14786419.2010.551755
80. Inbaneson, S. J., Sundaram, R., & Suganthi, P. (2012). In vitro antiplasmodial effect of ethanolic extracts of traditional medicinal plant Ocimum species against Plasmodium falciparum. Asian Pacific journal of tropical medicine, 5(2), 103–106. https://doi.org/10.1016/S1995-7645(12)60004-2
81. Gbolade AA, Lockwood GB. Toxicity of Ocimum sanctum L. essential oil to Aedes aegypti Larvae and its chemical composition. J Essent Oil-Bear Plants. 2008
82. Agarwal, P., & Nagesh, L. (2011). Comparative evaluation of efficacy of 0.2% Chlorhexidine, Listerine and Tulsi extract mouth rinses on salivary Streptococcus mutans count of high school children--RCT. Contemporary clinical trials, 32(6), 802–808. https://doi.org/10.1016/j.cct.2011.06.007
83. Malhotra, R., Grover, V., Kapoor, A., & Saxena, D. (2011). Comparison of the effectiveness of a commercially available herbal mouthrinse with chlorhexidine gluconate at the clinical and patient level. Journal of Indian Society of Periodontology, 15(4), 349–352. https://doi.org/10.4103/0972-124X.92567
84. Kukreja BJ, Dodwad V. Herbal mouthwashes-A gift of nature. Int J Pharma Bio Sci. 2012;3:46–52.
85. Singh P, Mittal VK, Gupta SC. Trace elements in typical herbs as an indicator of environmental pollution. Indian J Environ Prot. 2003;23:1114–9
86. United Nations Conference on Trade and Development. Trade and Environment Review 2013. Wake Up Before It Is Too Late: Make Agriculture Truly Sustainable Now for Food Security in a Changing Climate; Geneva, United Nations. 2013